Abstract
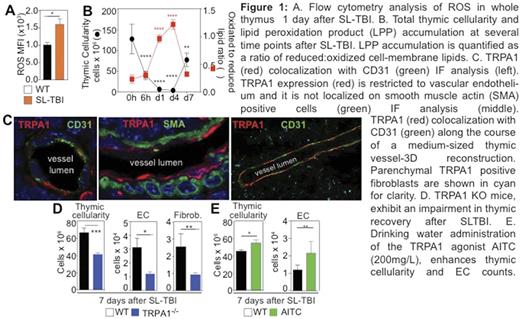
The thymus is extremely sensitive to exogenous insults but has a remarkable capacity to regenerate which is lost with age. Reactive oxygen species (ROS) accumulate early after tissue damage and despite their toxic potential, ROS and their byproducts (such as lipid peroxidation products-LPPs) can act as regeneration signals by activating membrane or intracellular sensors and subsequent stress-response signalling pathways. Using Sublethal Total Body Irradiation (SL-TBI) as a model of acute thymic injury, we found a rapid accumulation of thymic ROS as well as lipid peroxidation products on cell membranes after SLTBI (Figure 1A&B).
The damage-sensing ion channel Transient Receptor Potential cation channel family A member 1 (TRPA1) represents one of the major damage sensing receptors that can mediate cellular responses to oxidative stress mediators, such as LPPs. Using immunofluorescence (IF) microscopy we found that TRPA1 is enriched in the thymic medulla. Interestingly, although TRPA1 has been classically identified in nociceptive fibers, the major TRPA1 expressing structures in the thymus were not nerve fiber terminals, but primarily thymic endothelial cells (Figure1C), fibroblasts and subsets of epithelial cells. We have recently demonstrated that thymic endothelial cells can regulate regeneration through secretion of BMP-4, which can enhance Foxn1 expression and proliferation of thymic epithelial cells. In order to assess the functional role of TRPA1 in thymic regeneration after injury, we utilized TRPA1 knockout (TRPA1-/-) mice and quantified thymic reconstitution after SL-TBI. TRPA1-/- mice had significantly lower thymic cellularity compared to their age- and sex-matched WT controls, suggesting an association between TRPA1 deficiency and delayed endogenous thymic recovery (Figure 1D). The major deficit in thymocyte counts primarily affected double negative-4 (DN4), double positive (DP) and CD4+ single positive (SP-CD4+) thymocyte numbers. The thymic stroma of TRPA1-/- mice had lower endothelial cell and fibroblast counts (Figure 1D). In accordance with these findings drinking water administration of the TRPA1 agonist Allyl-Isothiocyanate (AITC), resulted in enhanced thymic regeneration after radiation exposure. Besides its positive effects on thymocyte counts, AITC significantly augmented endothelial cell counts after irradiation (Figure 1E).
In conclusion these results suggest that TRPA1 plays a non-redundant role in thymic regeneration and that exogenous TRPA1 stimulation can enhance immune recovery after damage.
van den Brink: Seres: Research Funding; Jazz Pharmaceuticals: Consultancy; PureTech Health: Consultancy; Therakos Institute: Other: Speaking engagement.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal